Part 3 Preventing Corrosion – Glass Versus Stainless Steel
There are several methods for addressing different forms of corrosion that occur within commercial hot water systems.
Adding tin to brass, for example, creates Dezincification Resistant Brass (DZR). Fittings manufactured from this alloy will be marked in the UK with the letters CR (Corrosion Resistant) or DZR (dezincification resistant).
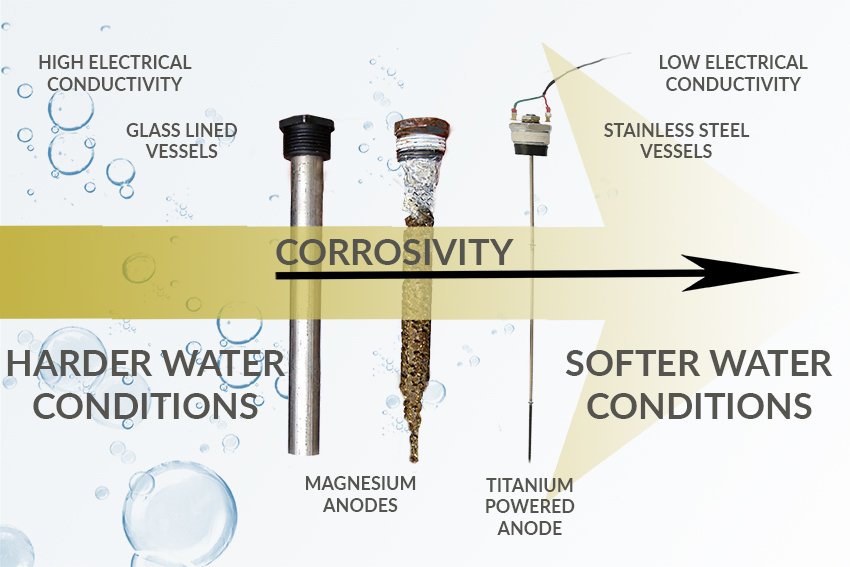
Commercial glass-lined steel water heaters and tanks are also attractive propositions as the glass is, given the right conditions, generally resistant to attack from most chemicals and corrosive materials. The glass is composed of several oxides and silicates blended and heated to the melting point. The first coat of glass is applied to develop a chemical bond with the steel of the tank and has limited corrosion resistance. After the ground coat, layers of chemically resistant glass are then added to create a smooth surface which is more resistant and easier to clean, making them popular in harder water areas.
However, not all glass-lining processes are equal with some being prone to developing microscopic cracks in the lining of the vessels. This means that small areas of the steel cylinder shell may get exposed to water, and there is an opportunity for corrosion to take hold.
Galvanic corrosion in water heaters and tanks is typically managed using cathodic anodes made of magnesium that offer a target for oxidisation in lieu of the steel shell, corroding or being ‘sacrificed’ first. Regular checks and replacement of anodes is critical in delivering ongoing protection from corrosion. Protective epoxy and plastic coatings can also be used to reduce corrosion by preventing conductivity from the water to the metal.
 The attack rate is determined by temperature, duration, and the concentration of reagents, for example, the presence of fluorides at any temperature will corrode a glass-lining.
The attack rate is determined by temperature, duration, and the concentration of reagents, for example, the presence of fluorides at any temperature will corrode a glass-lining.
In naturally soft water conditions, despite the use of sacrificial anodes, glass-lined vessels can rapidly succumb to critical corrosive damage. Due to a lack of dissolved metal ions, the purer soft water has low electrical conductivity, so the electrical flow from the anode to the cathode through the water is reduced. This adversely impacts the chemical reaction between sacrificial anode and cylinder shell, reducing the protection. In such cases, when the sacrificial anode is inspected its condition can be extremely good, but this is likely to be a strong indicator that the anode is failing in its role, meaning the water heater itself is being corroded.
The usual alternative to the sacrificial anode is the powered anode. Often made of titanium, the powered anode rather than giving up its own electrons and producing an electrolytic current produces a very low current in the water. This should provide a similar protective effect for a heater’s steel shell but without corroding the anode. However, in soft water areas, a powered anode may still not have a protective effect, as the conductivity required of the water by the anode is too poor.
For this reason, commercial hot water systems in Scotland, south-west and north-west of England and the west of Wales where water is particularly soft will typically need to employ a stainless-steel appliance. Better able to stand up to both water-side and combustion-side assaults, a stainless-steel heater is less susceptible to corrosion, due to the composition of the alloys, which create a protective oxide barrier on the waterside that naturally helps prevents corrosion, even when temperatures increase. Able to withstand higher temperature water (in excess of 80°C) than glass-lined appliances, stainless-steel lends itself to solar thermal and wider commercial applications.
Typically, stainless-steel will be used in indirect DHW heaters, where the internal heat transfer coil is connected to a boiler or a solar thermal collector loop; and in condensing water heaters where the push for higher efficiency condensing units has led to stainless steel being used to construct the heat exchangers. To achieve high efficiencies, flue gases must be cooled below the dew point to release the latent heat of condensation. With very low pH and high acidity, the resultant condensate would have a highly corrosive impact on the surfaces of the heat exchanger which regular steel or copper would struggle to withstand for any length of time.
Stainless steel is therefore preferable due to its versatility for creating intricate forms required by the heat exchanger and its high resistance to corrosion. This does mean stainless steel construction is typically more expensive, due to both higher material and manufacturing costs. Commercially, the investment is worthwhile, especially in soft water areas where, compared with replacement costs as a result of corrosion in glass-lined alternatives, stainless steel can prove far more cost-effective, with its quality reflected in the longer product warranties.
In the commercial world, domestic hot water (DHW) appliances are subjected to extremely hostile conditions, with high temperatures, thermal stress and flue gas condensate on the combustion side and oxygen, minerals and chemical attacks leading to potential corrosion on the waterside. Given this harsh daily treatment, regular servicing and maintenance are key if business-critical service is to be observed. Failure to descale, flush sediment, check anodes or test for corrosion will reduce the operational longevity of any appliance. In soft water areas, poor consideration of prevalent conditions and a lack of regular maintenance can reduce an appliance lifespan from years to a matter of months!
Correctly sizing, obtaining and then regularly servicing the right appliances and ancillaries for your application is critical if a commercial hot water system is to operate safely, efficiently and cost-effectively.
















